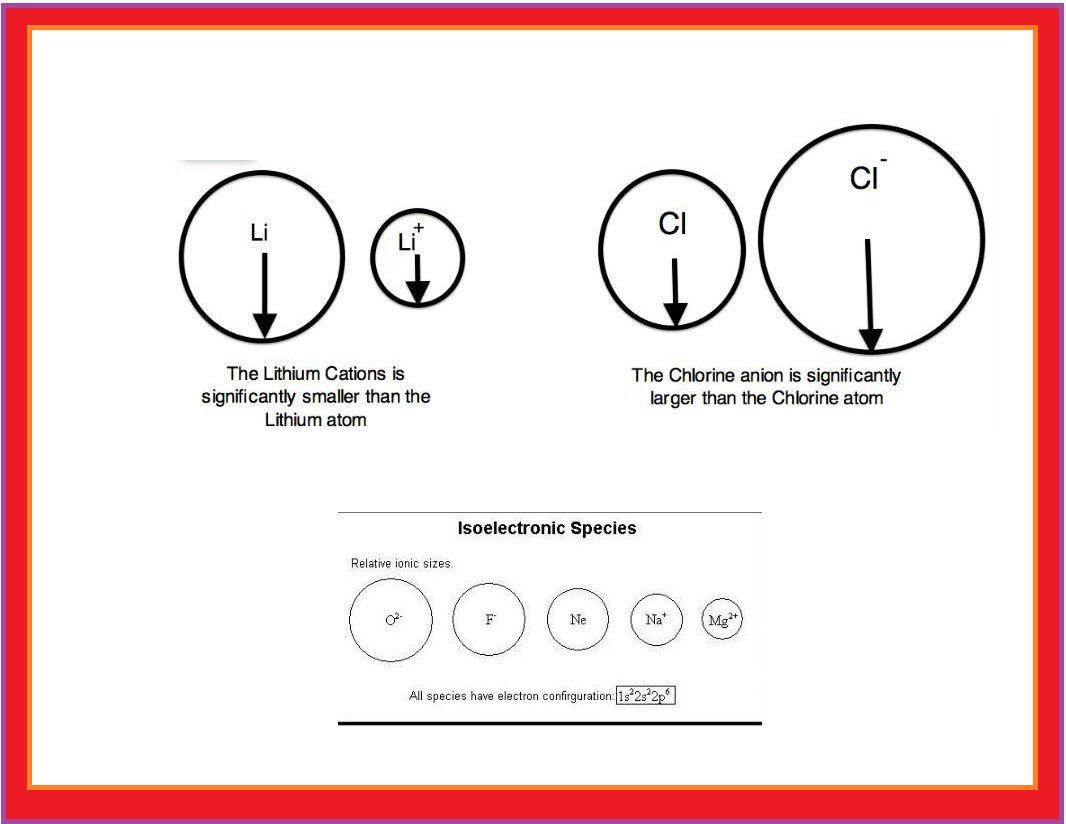
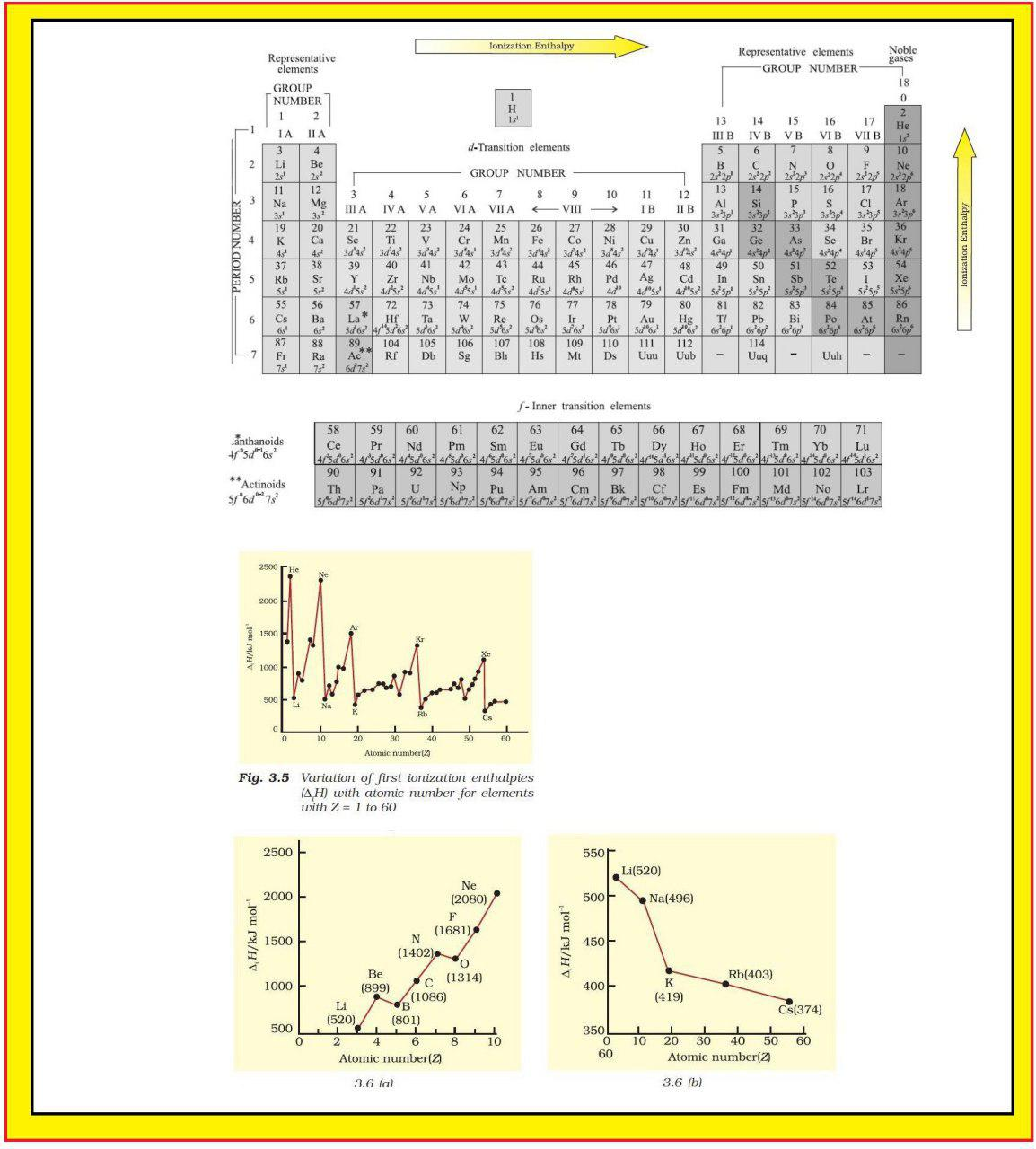
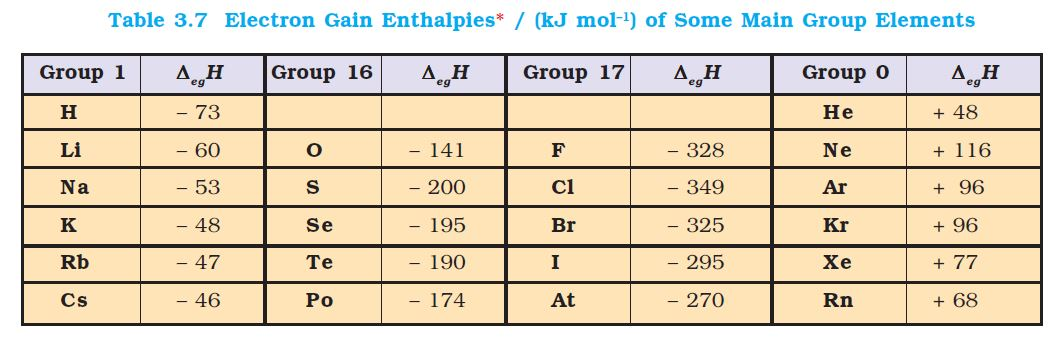
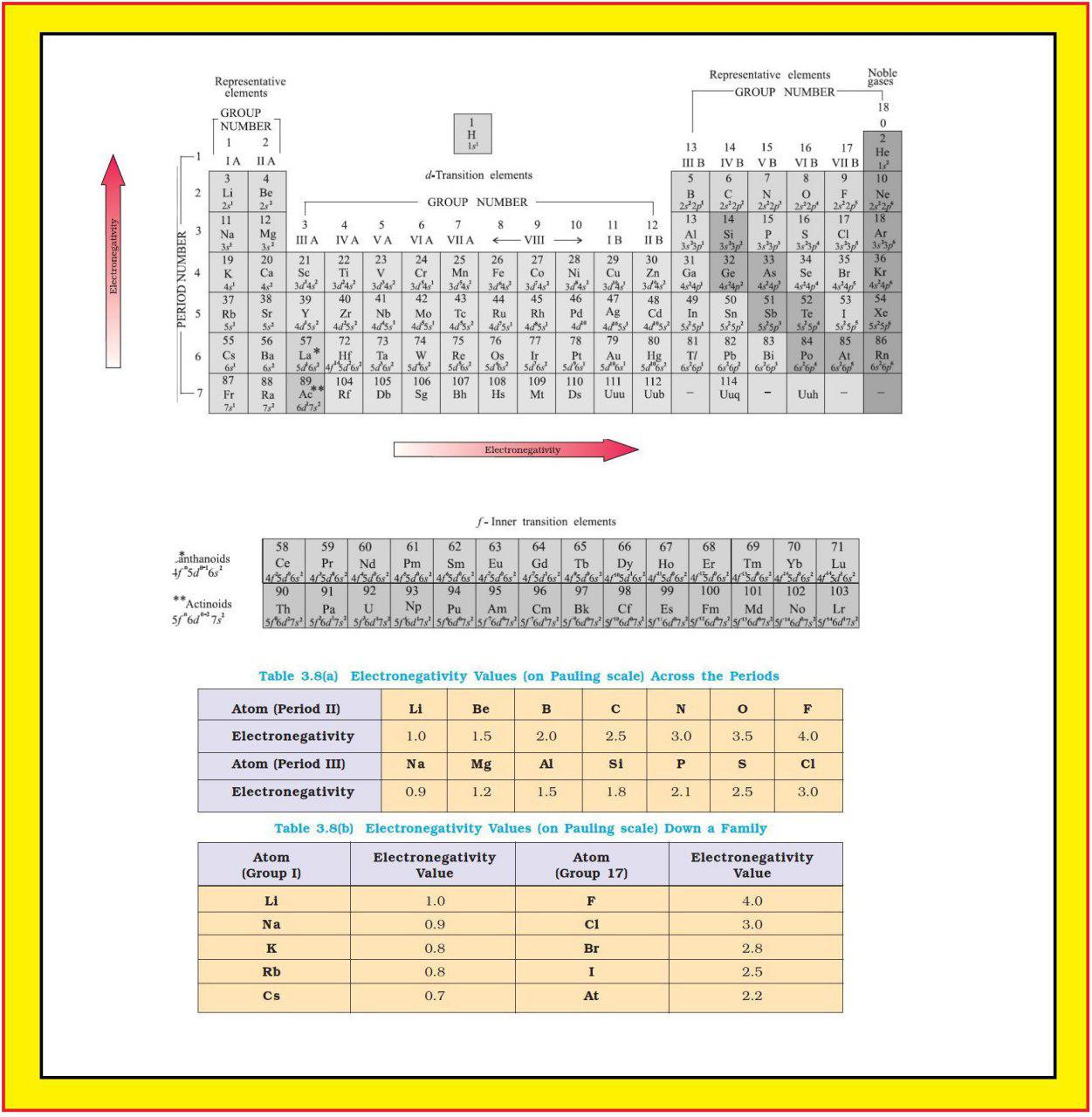
Atomic Radius :
`=>` Finding the size of an atom is a lot more complicated than measuring the radius of a ball.
● Firstly, because the size of an atom `(~ 1.2 Å` i.e., `1.2 × 10^(–10) m` in radius`)` is very small.
● Secondly, since the electron cloud surrounding the atom does not have a sharp boundary, the determination of the atomic size cannot be precise.
`=>` There is no practical way by which the size of an individual atom can be measured.
`=>` However, an estimate of the atomic size can be made by knowing the distance between the atoms in the combined state.
`=>` One practical approach to estimate the size of an atom of a non-metallic element is to measure the distance between two atoms when they are bound together by a single bond in a covalent molecule and from this value, the “Covalent Radius” of the element can be calculated.
● `text(Example :)` The bond distance in the chlorine molecule `(Cl_2)` is `198` pm and half this distance (`99` pm), is taken as the atomic radius of chlorine.
`=>` For metals, we define the term “Metallic Radius” which is taken as half the internuclear distance separating the metal cores in the metallic crystal.
`text(Example :)` The distance between two adjacent copper atoms in solid copper is `256` pm; hence the metallic radius of copper is assigned a value of `128` pm.
● For simplicity, we use the term Atomic Radius to refer to both covalent or metallic radius depending on whether the element is a non-metal or a metal.
● Atomic radii can be measured by `X`-ray or other spectroscopic methods.
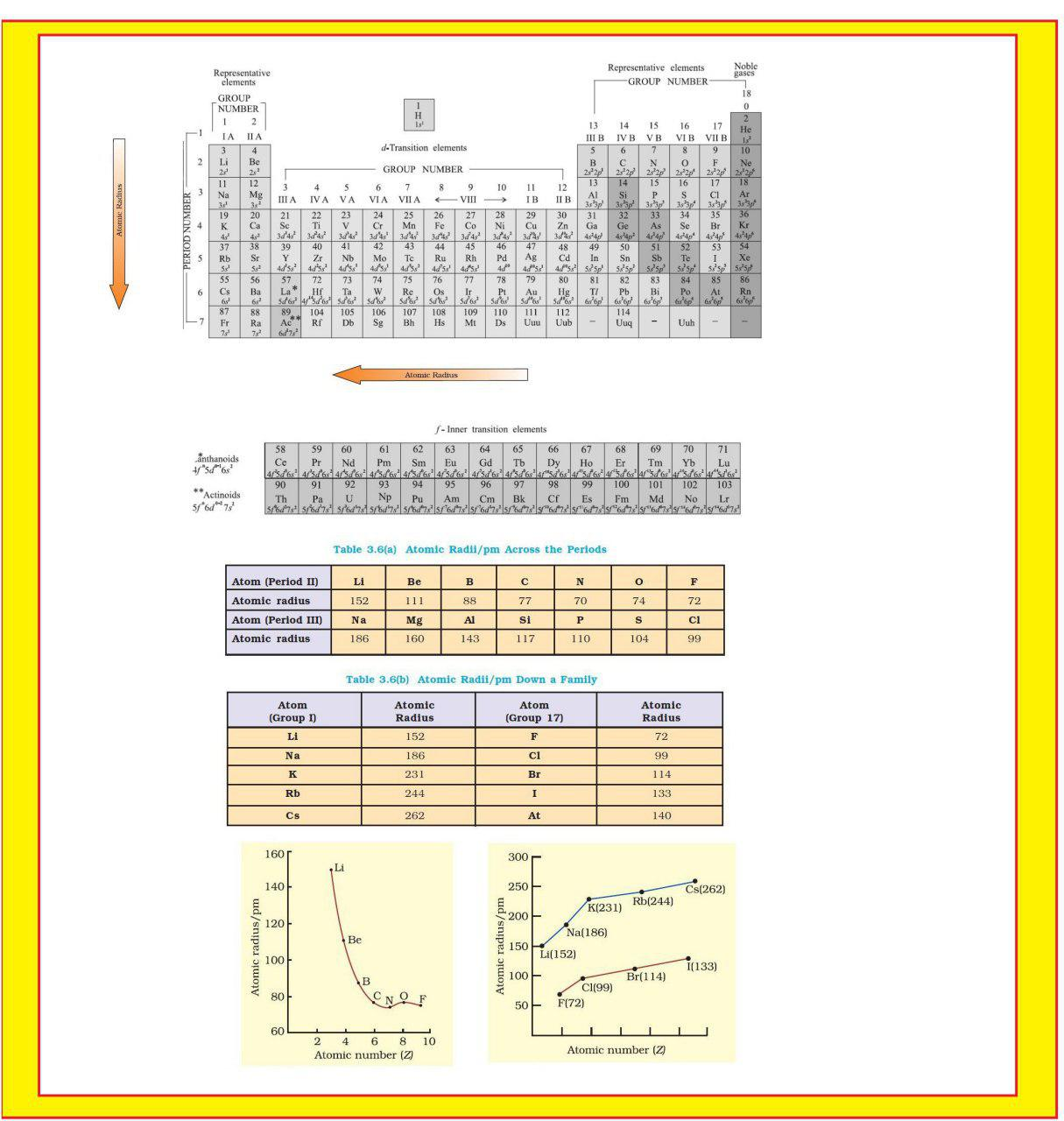
`=>` The atomic radii of a few elements are listed in Table 3.6.
`=>` Trends in atomic radius in terms of nuclear charge and energy level.
`=>` The atomic size generally decreases across a period as illustrated in Fig. 3.4(a) for the elements of the second period.
● It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus.
● Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number as illustrated in Fig. 3.4(b).
● For alkali metals and halogens, as we descend the groups, the principal quantum number (`n`) increases and the valence electrons are farther from the nucleus.
● This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus.
● Consequently the size of the atom increases as reflected in the atomic radii.
`text(Note :)` The atomic radii of noble gases are not considered here.
● Being monoatomic, their (non-bonded radii) values are very large.
● In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements.
● Firstly, because the size of an atom `(~ 1.2 Å` i.e., `1.2 × 10^(–10) m` in radius`)` is very small.
● Secondly, since the electron cloud surrounding the atom does not have a sharp boundary, the determination of the atomic size cannot be precise.
`=>` There is no practical way by which the size of an individual atom can be measured.
`=>` However, an estimate of the atomic size can be made by knowing the distance between the atoms in the combined state.
`=>` One practical approach to estimate the size of an atom of a non-metallic element is to measure the distance between two atoms when they are bound together by a single bond in a covalent molecule and from this value, the “Covalent Radius” of the element can be calculated.
● `text(Example :)` The bond distance in the chlorine molecule `(Cl_2)` is `198` pm and half this distance (`99` pm), is taken as the atomic radius of chlorine.
`=>` For metals, we define the term “Metallic Radius” which is taken as half the internuclear distance separating the metal cores in the metallic crystal.
`text(Example :)` The distance between two adjacent copper atoms in solid copper is `256` pm; hence the metallic radius of copper is assigned a value of `128` pm.
● For simplicity, we use the term Atomic Radius to refer to both covalent or metallic radius depending on whether the element is a non-metal or a metal.
● Atomic radii can be measured by `X`-ray or other spectroscopic methods.
`=>` The atomic radii of a few elements are listed in Table 3.6.
`=>` Trends in atomic radius in terms of nuclear charge and energy level.
`=>` The atomic size generally decreases across a period as illustrated in Fig. 3.4(a) for the elements of the second period.
● It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus.
● Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number as illustrated in Fig. 3.4(b).
● For alkali metals and halogens, as we descend the groups, the principal quantum number (`n`) increases and the valence electrons are farther from the nucleus.
● This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus.
● Consequently the size of the atom increases as reflected in the atomic radii.
`text(Note :)` The atomic radii of noble gases are not considered here.
● Being monoatomic, their (non-bonded radii) values are very large.
● In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements.